Myelodysplastic Neoplasms (MDS) are hypothesized to re-model their bone marrow (BM) microenvironment to re-inforce conditions for their propagation. In this study, we investigated interactions between MDS cells and the BM niche at single cell level in a MDS patient-derived xenograft model (PDX) as well as primary human MDS and healthy (HY) bone marrow (BM samples in order to identify cues of disturbed crosstalk between MDS hematopoiesis and the BM microenvironment.
In the PDX model, we analyzed >13,000 cells of n=15 different murine niche cell populations after long-term (>24 weeks) exposure to n=3 MDS patient samples versus n=2 age-matched HY human grafts. Subsequently, we analyzed over 24,000 primary human BM cells enriched for the non-hematopoietic compartment by using whole bone fragments from n=8 MDS patients and n=7 HY age-matched donors. All cells were analyzed with combined 10x single cell RNA sequencing and human “Cellular Indexing of the Transcriptome and Epitopes by Sequencing” (CITE Seq). Results were validated by flow cytometry and gene expression analyses.
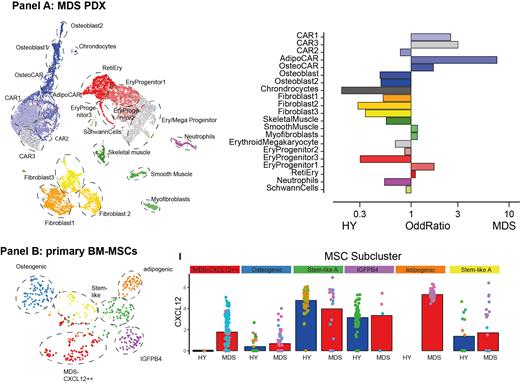
Mesenchymal cell (MSC) subpopulations from PDX mice exposed to MDS versus HY hematopoiesis could be subdivided into distinct “Cxcl12+ abundant reticular” (CAR) cells with osteogenic and adipogenic differentiation trajectories. Various CAR cell populations displayed an increased frequency in MDS transplanted mice as compared to PDX transplanted with HY grafts ( Panel A). Upon contact with hematopoietic MDS cells, CAR cell populations overexpressed numerous genes involved in extracellular matrix reorganization and hematopoietic supporting factors, such as Cxcl12, Il7, Lox and Loxl1 as compared to mice bearing HY grafts.
In situ, BM cells from MDS patients showed highly heterogeneous MSC subpopulations with similar differentiation trajectories to the ones observed in our PDX models on a patient-individual level. We detected two non-hematopoietic cell populations, designated as endothelial cells (ECs) and MSCs characterized by expression of CXCL12, IGFBP5 or COL1A2 and quantification with epitope sequencing (CITE Seq) with mutually exclusive detection of CD31 and CD271 expression (n=581 cells in total, Panel B). In n=3 MDS patients, we identified an MDS-specific MSC subpopulation with high IGFPB4 and CXCL12 expression as well as overexpression of KITLG and IL7. This suggests that this altered specialized secretory mesenchymal state is present in a subset of MDS patients and may support the malignant hematopoietic clones by overexpression of key hematopoietic factors.
Interactome analyses using spatial receptor:ligand bioinformatic tools such as CellphoneDB, RNAMagnet and omnipath further confirmed that stromal cells alter their cellular profile and secretory behavior in response to MDS hematopoiesis.
In conclusion, we show that murine BM stroma is re-programmed by exposure to human MDS xenografts to upregulate extracellular matrix (ECM) re-modeling and HSC supporting factors. In native human MDS BM in vivo, rare primary mesenchymal cells are re-programmed to increase hematopoietic support in a patient-individual manner pointing to altered MSC subpopulations with increased growth factor expression profiles in a subgroup of MDS patients.
Disclosures
Nowak:Affimed: Research Funding; Pharmaxis: Research Funding; Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal